About NEXPLANON®
NEXPLANON® is a sub dermal contraceptive implant that lasts for up to 3 years and is over 99% effective1
Indication: NEXPLANON is for contraception. Safety and efficacy have been established in women between 18 and 40 years of age
NEXPLANON is a method of long-acting reversible contraception (LARC).1
Progestogen only
Containing only the progestogen etonogestrel,1 NEXPLANON is suitable for a wide range of women. The most recent UK Medical Eligibility Criteria for contraceptive use should be used when assessing a woman’s eligibility for any contraceptive method including the progestogen-only implant.2
References
- NEXPLANON 68 mg implant for subdermal use – Summary of Product Characteristics.
- Faculty of Sexual & Reproductive Healthcare (FSRH). The UK Medical Eligibility Criteria for Contraceptive Use. Available from https://www.fsrh.org/ukmec/.
- Glasier A. Contraception 2002; 65(1): 29–37.
- Davies GC et al. Release characteristics, ovarian activity and menstrual bleeding pattern with a single contraceptive implant releasing 3-ketodesogestrel. Contraception 1993; 47(3): 251-61.
- Trussel J. Contraception 2011; 83(5): 397–404.
- NICE Clinical Guideline 30. Long-acting reversible contraception. London.
Many studies referenced here are about IMPLANON. NEXPLANON is bioequivalent to IMPLANON.
Supporting documentation
Prescribing Information | Summary of Product Characteristics | Patient Information Leaflet
By clicking the above links you will leave the Organon website and be taken to external websites.
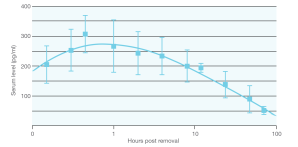
 Each single, flexible rod releases a continual daily dose of the progestogen etonogestrel for up to 3 years.1
Each single, flexible rod releases a continual daily dose of the progestogen etonogestrel for up to 3 years.1
